
Physician
trusted

Highest level of accuracy available

Antibiotic resistance panel included

COLA Accredited & CLIA Certified Lab Partners

Telehealth
Compatible

HSA/FSA
Accepted
Not available in HI, AK, and NY
ReliaHealth is the New Gold Standard for Clinical Laboratory Tests
Our comprehensive at-home test kits provide you with an accurate, easy solution to understanding your health and empower you to make medically-informed decisions for the best course of treatment so you feel better faster.
Faster & Easier
- No doctor's appointment
needed - Free Overnight Shipping
- Results in 48 Hours of
receiving your sample
More Accurate
- Highest level of clinical
accuracy available - DNA amplification technology
allows for early detection - Tests for 15+ types
of bacteria
The Right Treatment
- The only mail-in test kit that
includes antibiotic resistance data - Provides 20+ antibiotic
resistance markers - Facilitates personalized
treatment options
Accuracy Ratings
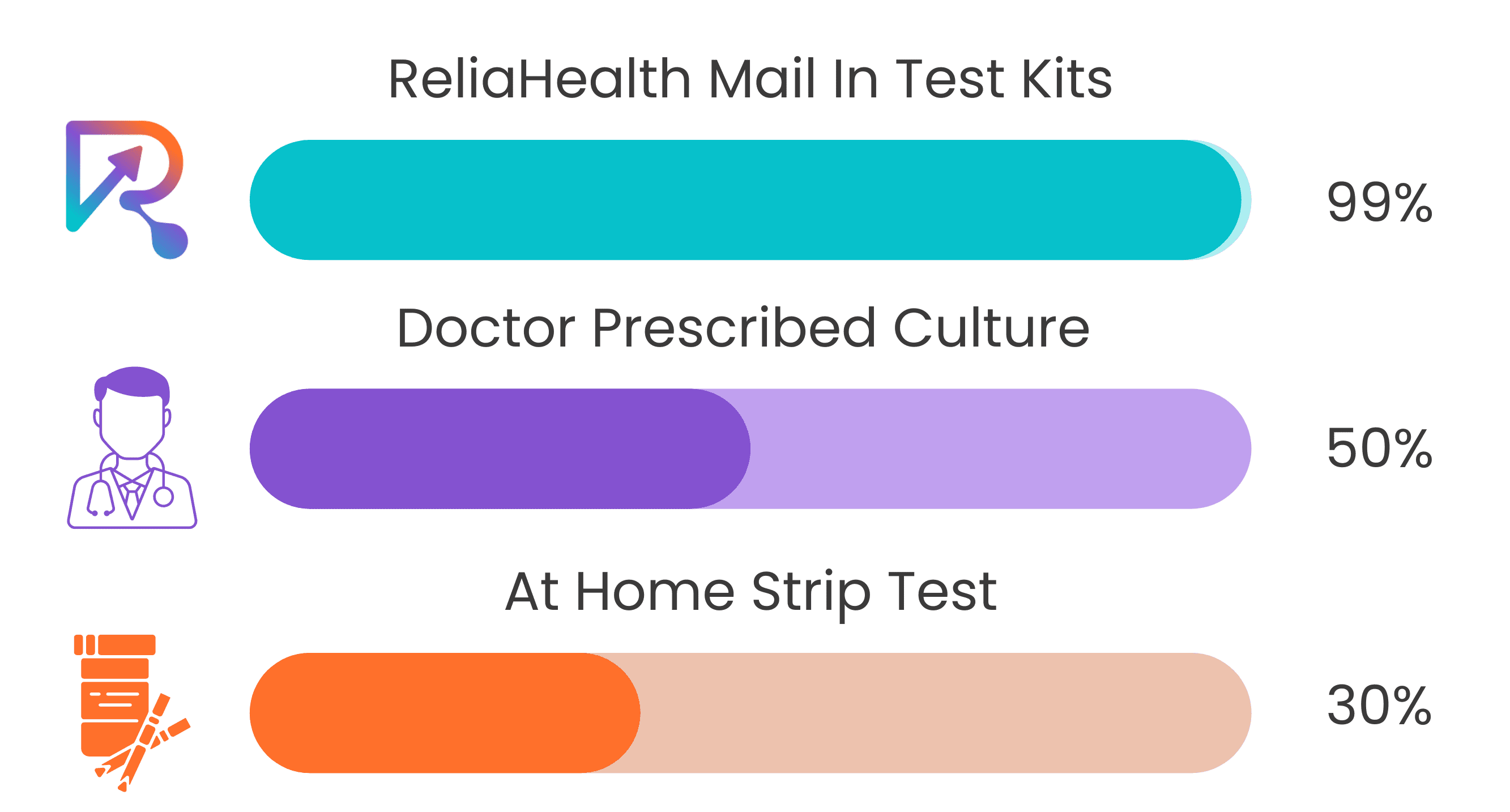
How Do ReliaHealth Tests Compare?
Infections can’t be properly treated without knowing the specific cause. ReliaHealth PCR tests are highly sensitive and pick up on biomarkers often missed by the current industry-accepted tests. Every sample is analyzed utilizing state of the art technology in a CLIA Certified and COLA Accredited laboratory. Our sample analysis process identifies the exact cause of your infection and provides guidance on how to effectively treat it- allowing you to skip the doctor’s office.

ReliaHealth Mail In Test Kits
- No doctor’s appointment required
- Antibiotic resistance panel included
- Results in 24-48 hours

Doctor Prescribed Culture
- Required in-office doctor’s visit
- Results in 7-10 days
- No antibiotic resistance panel included
Strip Test
- Lowest accuracy ratings
- Not accepted for medical treatment
- No antibiotic resistance panel included
Advanced Laboratory Testing from Anywhere
ReliaHealth provides the most comprehensive mail in test kits available, testing up to 5 times the number of pathogens for accurate, specific diagnosis. Results include antibiotic resistance analysis of the infection cause, allowing for an individualized treatment plan to address your condition. Test results are electronically delivered to you via our private patient portal to make it easier than ever for you to take back control of your health.
1. Order Kit
Order one of our test kits online and it will be delivered as quickly as within 24 hours to your doorstep.

2. Ship Sample
Follow the instructions in your test kit, then mail your sample using the provided box and prepaid shipping label.

3. Get Results
View your lab results online in our private patient portal.

4. Get Prescription
Once you obtain your test results, you can set up an online Telehealth appointment. The healthcare provider will review your results online and prescribe the correct form of treatment.
3 Reasons to Choose ReliaHealth
Discover the features of ReliaHealth’s fast, accurate, at-home test kits.

The Lab
The ReliaHealth specialized laboratory partners are able to effectively identify and scan for antibiotic-resistant bacteria and fungi to provide you with a proper treatment plan for your specific infection. Our laboratory partners are HIPAA Compliant and take the utmost care to ensure your privacy. Our custom in-house patient portal utilizes high levels of encryption to store your data securely.
- No extra cost or co-pays required.
- We accept FSA and HSA cards
- DNA Amplification technology for faster, more accurate results


Shop High Accuracy At-Home Test Kits
FAQ
Unlike other tests such as culture tests which have 50% accuracy or test
strips which have 30% accuracy, ReliaHealth’s RT-PCR (Real Time Polymerase
Chain Reaction) also known as NAAT tests (Nucleic Acid Amplification Tests) are
98% accurate and can find evidence of disease in the earliest stages of
infection.
RT-PCR/NAAT tests work because they identify small amounts of DNA or RNA in the
test samples. They can, therefore, be used to identify bacteria, viruses, and
other pathogens even when the material of interest is present in very small
amounts.
Other tests may miss early signs of disease because there aren’t enough
viruses, bacteria, or other pathogens in the sample, or your body hasn’t had
enough time to develop an antibody response. The patient is left suffering with
symptoms for extended periods of time until an infection can be determined.
With ReliaHealth’s RT-PCR technology, you don’t have to wait.
Polymerase Chain Reaction (PCR) technology was invented by Kary B. Mullis, an American biochemist. Mullis developed the PCR technique in 1983, and he was awarded the Nobel Prize in Chemistry in 1993 for this groundbreaking invention. Kary B. Mullis’ development of PCR revolutionized molecular biology and diagnostics. PCR has become an essential tool in various scientific fields, including genetics, forensics, and medical diagnostics. The ability to amplify and analyze DNA rapidly and efficiently has had a profound impact on scientific research and clinical diagnostics. References
Antibiotics are medicines used to fight bacterial infections. Overtime, bacteria has developed resistance to antibiotics due to the over-prescription of these medicines in both humans and livestock.
An antibiotic resistance panel can help find out which antibiotic the bacteria in your UTI or Nail Infection is resistant to so that you are not prescribed the incorrect treatment against the bacteria.
This is why ReliaHealth 2-in-1 UTI Test and the ReliaHealth 3-in-1 Nail Test provides an antibiotic resistance panel which detects 24 antibiotic resistance genes found in the strains of bacteria that are most commonly the cause of UTI’s and Nail Infections.
This allows for the correct antibiotic prescription to fight off your infection and get rid of it for good.
Other names: antibiotic susceptibility test, antibiotic sensitivity testing, antimicrobial susceptibility test
No. Antibiotic resistance develops in the bacteria, not your body. Whether you’ve taken antibiotics before or not, the bacteria itself such as E.coli already have developed hundreds of resistant strains.
Test kits are shipped directly to your door using free overnight shipping or the fastest available shipping option based on your location. Test kits typically arrive within 1-3 business days. We also provide a prepaid shipping label on the return box so your return of the sample is seamless and arrives at the lab within a few days.
Our lab partners test for 17 or more pathogens and over 20 antibiotic resistance markers. Patients will be able to see exactly the bacteria and/or fungi found in their sample and the antibiotics that are suitable or unsuitable to treat the infection.
With ReliaHealth test kits your results are provided 1-2 business days after receiving your sample.
The Commission on Office Laboratory Accreditation (COLA) is a private, non-profit accreditation organization that helps laboratories meet CLIA and other regulatory requirements. COLA Inc. accredits more than 7,400 medical laboratories and provides the clinical laboratory with a program of education and accreditation.
COLA prepares laboratories to meet both federal and state regulatory requirements. This involves recording laboratory self-assessments, performing on-site surveys, documenting corrective action plans for non-compliant incidents, and monitoring proficiency testing. COLA accreditation is renewed every 2 years. https://www.cola.org/about/
The Centers for Medicare & Medicaid Services (CMS) regulates all laboratory testing (except research) performed on humans in the U.S. through the Clinical Laboratory Improvement Amendments (CLIA). In total, CLIA covers approximately 320,000 laboratory entities. The Division of Clinical Laboratory Improvement & Quality, within the Quality, Safety & Oversight Group, under the Center for Clinical Standards and Quality (CCSQ) has the responsibility for implementing the CLIA Program. https://www.cms.gov/medicare/quality/clinical-laboratory-improvement-amendments?redirect=/CLIA/
Your sample analysis and antibiotic sensitivity results can be downloaded directly from our HIPAA Compliant, private patient portal. Simply download and email your results to your doctor for treatment. You can also set up a Telehealth appointment with our partner Sesame by clicking here.
A same-day telehealth appointment is easy to set up with our partner Sesame. Click below to schedule a consultation today!




